Synthetic Cannabinoids Lead to Vehicular Homicide
Reproduced with permission from ToxTalk Volume 37, Issue 4, pages 14-17, December 2013
No, Dorothy, synthetic cannabinoids are not just strong marijuana! This is the message we, as toxicologists, must repeatedly tell the media and inform the public. Just because some of these drugs can be purchased over-the-counter as bath salts, it does not mean that they are free of highly toxic side effects and the capacity to cause death both directly and indirectly. This case involved the synthetic cannabinoid, XLR11, an aminoalkylindoles, the largest group of synthetic cannabinoids among the 7 major structural groups often used to describe the chemical structures of synthetic cannabinoids. These groups include: naphthoylindoles (JWH-018, JWH-073, and JWH-398), naphthoylmethylindoles, naphthoylpyrroles, naphthylmethylindines, phenylacetylindoles (or benzoylindoles) (e.g., JWH-250), cyclohexylphenols (e.g., CP 47,497), and classical cannabinoids (e.g., HU-210). Some scientist break the seven structural groups listed above into three groups: classical cannabinoids, cyclohexylphenols and aminoalkylindoles.
Case Study
A group of 6 young men and women in their mid-20s were out socializing one evening. On their way home, one of the passengers pulled out a bag containing a vegetable-like material described as a “synthetic cannabinoid.” She proceeded to roll a “joint” and began passing it around for her friends to share. The driver took 3 “hits” from the joint and passed it along to another friend.
After smoking the synthetic cannabinoid, the driver allegedly ran 3 stoplights and then, according to the police report … failed to negotiate a curve, crossed over the double yellow line in the middle of the roadway, crossed through the northbound lane, continued over the sidewalk, striking several vinyl posts. According to the accident reconstruction report, “… there was no physical evidence on the road that indicated the driver attempted to negotiate the curve… no physical evidence of a critical speed yaw, no scuffing from rotation and no skid marks that would have indicated that the driver took any defensive or protective action prior to the impact.
The accident reconstruction report indicated that the movement of the car, just prior to hitting the tree, was pretty much a straight line from its position where it left the bend in the highway till the time the car came to rest, lodged against the tree. One survivor testified at the grand jury hearing that the driver was unable to hear or respond to the shouts of his name from his fellow passengers. This most likely was due to a continued decrease in his level of consciousness, due to the intensifying effect of the synthetic cannabinoid. Instead of slowing down, at one time prior to the crash, one grand jury witness testified that she perceived the car speeding up. This speeding up most likely represented a further decrease in consciousness as the driver passed from semi-consciousness to unconsciousness, where he was no longer able to support his body in any way and his center of gravity changed, as he slumped forward on the steering wheel causing his foot to further depress the accelerator pedal.
The witness who testified at the grand jury hearing testified that she fell asleep following one “hit” of the synthetic cannabinoid, and the driver inhaled at least 3 hits of the same synthetic cannabinoid.
The driver exhibited a continuum of descending levels of consciousness which culminated in his inability to respond to commands or control the motor vehicle he was driving. This is a very different scenario from simple impairment which most often presents itself with a driver weaving from left to right or crossing over a white or yellow line on the highway. The driver did not weave while driving, instead, he appears to have been frozen behind the wheel and unable to move, steer, brake or take any other defensive action which might have averted the collision with the tree. The lack of skidmarks or any indications of the driver’s attempt to control the vehicle were absent, indicating that the driver was not conscious during the time his vehicle left the road and headed for the tree. This is reinforced by the driver’s inability to respond to his name or to recognize the gravity of the impending collision with the tree.
The crash resulted in the deaths of 2 passengers and caused one young woman to become paralyzed from the waist down, a tragic ending for a group of friends out to enjoy themselves for the evening. The driver has been indicted for vehicular homicide.
Why is smoking synthetic cannabinoids is more dangerous than smoking marijuana?
The structures of synthetic cannabinoids differ markedly from the structures of naturally-occurring cannabinoids that are found in the marijuana plant, Cannabis Sativa. Currently, most synthetic cannabinoids fall into one of seven major structural groups: naphthoylindoles (JWH-018, JWH-073, and JWH-398), naphthoylmethylindoles, naphthoylpyrroles, naphthylmethylindines, phenylacetylindoles (or benzoylindoles) (e.g., JWH-250), cyclohexylphenols (e.g., CP 47,497), and classical cannabinoids (e.g., HU-210). Some scientists classify the groups into three categories: classical cannabinoids, cyclohexylphenols and aminoalkylindoles, the largest group, and the group to which XLR11 belongs. In contrast to the naturally-occurring cannabinoids which have a dibenzopyran nucleus and contain no N in their structure, 4 of the remaining 6 classes are indole derivatives and the remaining 2 are naphthyl-derivatives. Using the 3-group classification, the structures of common synthetic cannabinoids are shown in the accompanying table, which was graciously provided by Heather L. Harris, MFS, JD, D-ABC, to whom I am very grateful.
The indoles are related to the structures of LSD and dimethyltryptamine (DMT) which most likely contributes to their increased potency and hallucinogenic effects, in comparison to naturally-occurring cannabinoids.
The names or designations of the synthetic cannabinoids frequently have been derived from their discoverer or manufacturer. HU-210 is named for Hebrew University, where it was synthesized by Rafael Mechoulam in the 1980s. The CP compounds, or cyclohexylphenols, were developed by Pfizer pharmaceutical company as pain relievers in the late 1970s and named CP for Charles Pfizer, with CP-47, 497 representing the prototypical drug. The class of aminoalkylindoles frequently bear the initials JWH, representing Clemson University Prof. John W. Huffmann who first developed the JWH series in the late 1990s. This series originally included JWH-018, JWH-073, and JWH-200. Another class of synthetic cannabinoids, the phenylacetyliIndoles, often bears the designation RCS, which stands for Research Chemical Suppliers, of which RCS-8 is a prototypical example. Other synthetic cannabinoids include the benzoylindoles, which may bear a designation beginning with AM, which stands for Alexandros Makryannis, a synthetic organic chemist. Typical synthetic cannabinoids from this series include AM-694, AM-2201 and AM-1221. Agents designated as “Win” such as Win-55,212-2, an aminoalkylindole, were developed by Winthrop Labs., which used to be known as Sterling-Winthrop Pharmaceutical Company. Winthrop went on to synthesize more than 100 aminoalkylindole derivatives.
If the structures of synthetic cannabinoids differ so much from marijuana, why are they called cannabinoids?
According to the federal analog act, a controlled substance may be described as an analog of another group of illicit drugs if:
- the chemical structure of which is substantially similar to the chemical structure of a controlled substance in schedule I or II, and
- which has a stimulant, depressant, or hallucinogenic effect on the central nervous system that is substantially similar to or greater than the stimulant, depressant, or hallucinogenic effect on the central nervous system of a controlled substance in schedule I or II or
- with respect to a particular person, which such person represents or intends to have a stimulant, depressant, or hallucinogenic effect on the central nervous system that is substantially similar to or greater than the stimulant, depressant, or hallucinogenic effect on the central nervous system of a controlled substance in schedule I or II.
In addition to binding to the CB-1 cannabinoid receptors in the brain, synthetic cannabinoids have a depressant, or hallucinogenic effect on the central nervous system that is substantially similar to or greater than the depressant, or hallucinogenic effect of THC on the central nervous system. Some have called this class of drugs synthetic marijuana, but this terminology should be avoided as it implies that these drugs are “just potent marijuana” and this could not be further from the truth!
And so my fellow forensic toxicologist colleagues, this is my story about the dangers of synthetic cannabinoids, and I’m sticking to it!
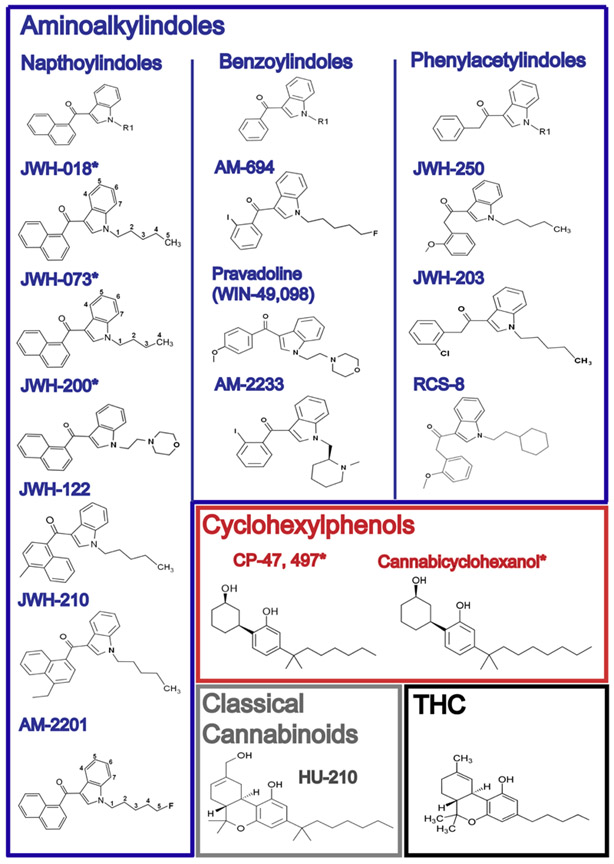
Modified from: Synthetic Cannabinoids: The Challenges of Testing for Designer Drugs
By Bridgit O. Crews, PhD Clinical Laboratory News: Volume 39, Number 2, February 2013.